This 60 year old healthy appearing woman with Lynch Syndrome was in excellent health until 2000 when she was diagnosed with endometrial cancer and underwent a total abdominal hysterectomy. She did well and did not have a recurrence.
In 2016 she was struck by a truck in New York City while walking along the street. She sustained a severe head injury. Fortunately, she was very close to a major medical center and received prompt excellent care. She underwent three craniotomies for control of a cerebral hemorrhage and subsequently required a shunt. She was in a coma for several weeks but recovered completely.
In 2017 after learning she had a family history of renal cancer she underwent genetic testing at Memorial Sloan Kettering Cancer Center. Her father and grandmother had kidney cancer. Her grandmother was diagnosed at an advanced stage and died of metastatic cancer. The patient does not know the type of kidney cancer. Her blood DNA sample identified a deletion of exons 9-10 in the MSH2 gene. This is diagnostic of Lynch Syndrome.
In September 2020 she underwent a colectomy for colon cancer. She has undergone regular imaging of her abdomen and pelvis since the endometrial and colon cancers were diagnosed and treated. She is free of recurrence. A routine September 2021 CT scan of the abdomen and pelvis indicated an area of thickening of the right bladder wall. An investigation resulted in the finding of a urinary tract infection. Flexible cystoscopy was normal. A subsequent urinalysis was normal. The patient is aware of the association of Lynch Syndrome and urothelial cancer and requested to have annual cystoscopy. The next cystoscopy was scheduled for the end of 2022.
In October 2022 she had gross hematuria. A CT scan indicated an 8 cm bladder mass located on the left lateral wall (Fig. 1). The upper urinary tract was normal. Due to various personal issues including a Covid infection she was not brought to the operating room until December 2022. A large, solitary, exophytic tumor was located on the left lateral wall above the left ureteral orifice. A transurethral resection was performed (Figs. 2–6). The pathology indicated a high grade urothelial carcinoma with focal squamous features and keratinization. There was invasion into the lamina propria. A separate specimen from the deeper portion of the resection indicated the same histology. There were a few strands of muscle, but the pathologist could not be certain whether this was muscularis mucosa or muscularis propria. In January 2023 she underwent a repeat resection of this area and there was ample muscularis mucosae in this tissue (Fig. 7, 8). There was no residual tumor. The rest of the bladder was carefully surveyed on both occasions with white light and narrow band imaging and was free of tumor. Thus, she had a large unifocal HG T1 urothelial carcinoma with squamous features with a second resection free of tumor.
Fig. 1
CT scan image indicating the large bladder tumor left wall.
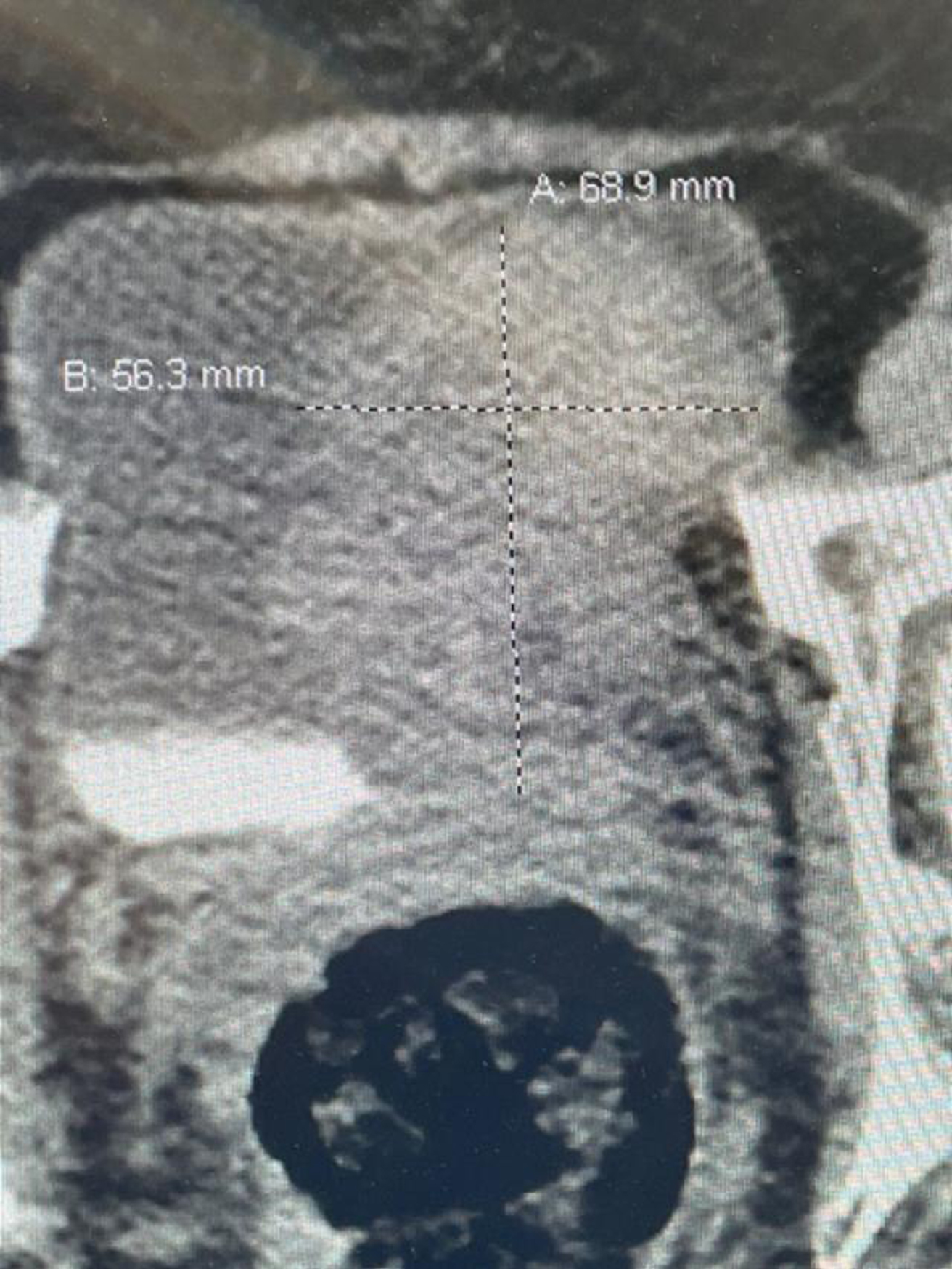
Fig. 2-3
Endoscopic appearance of the large solid appearing tumor and the loop to provide an idea of the size.
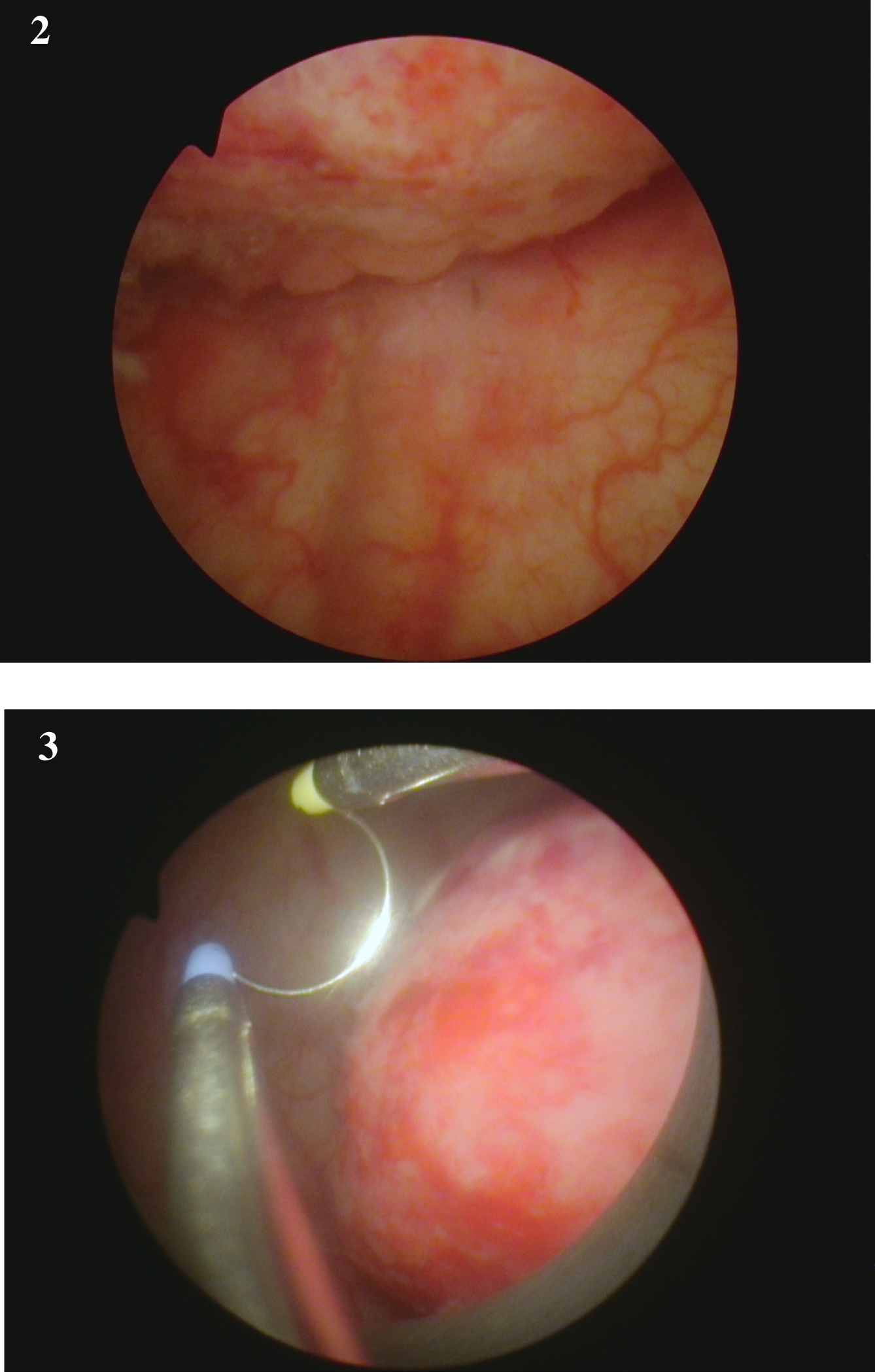
Fig. 4-6
are from the endoscopic resection of the large solid tumor. Grossly it appears to be an invasive cancer.
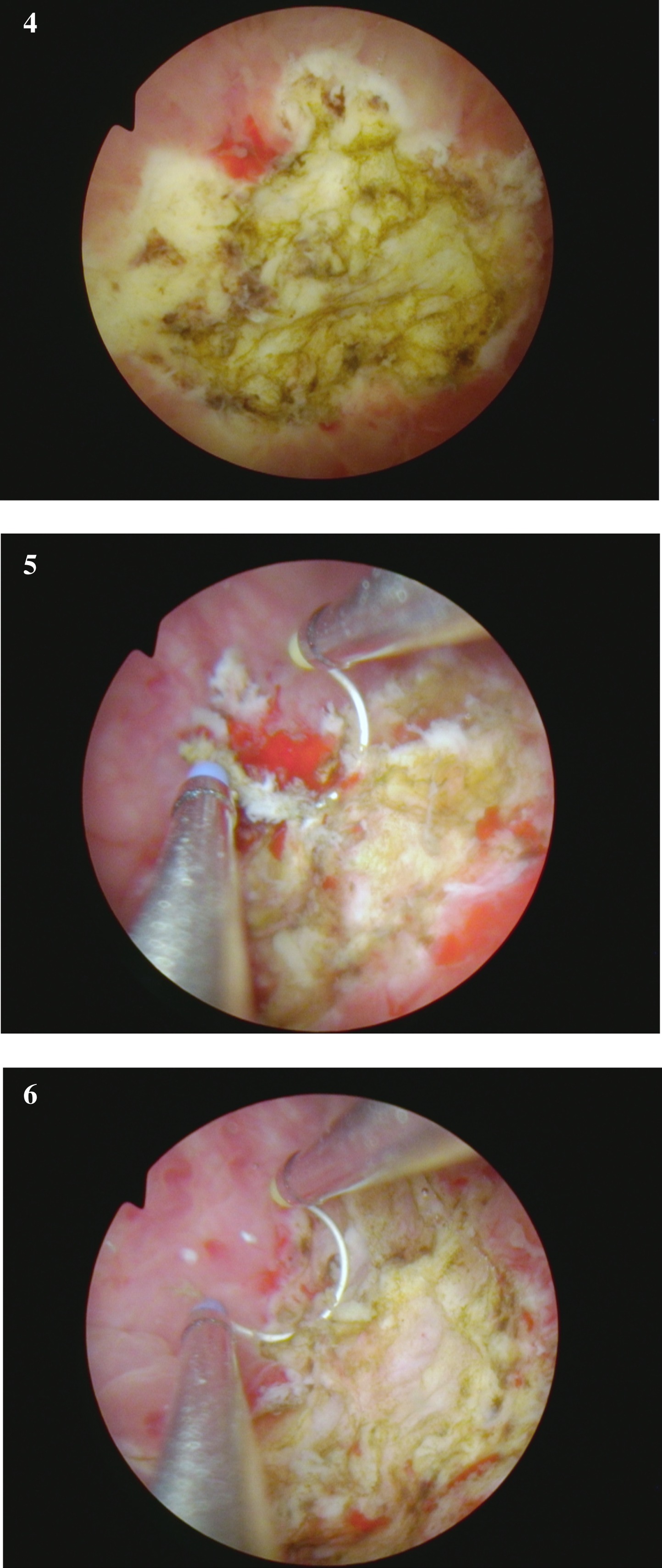
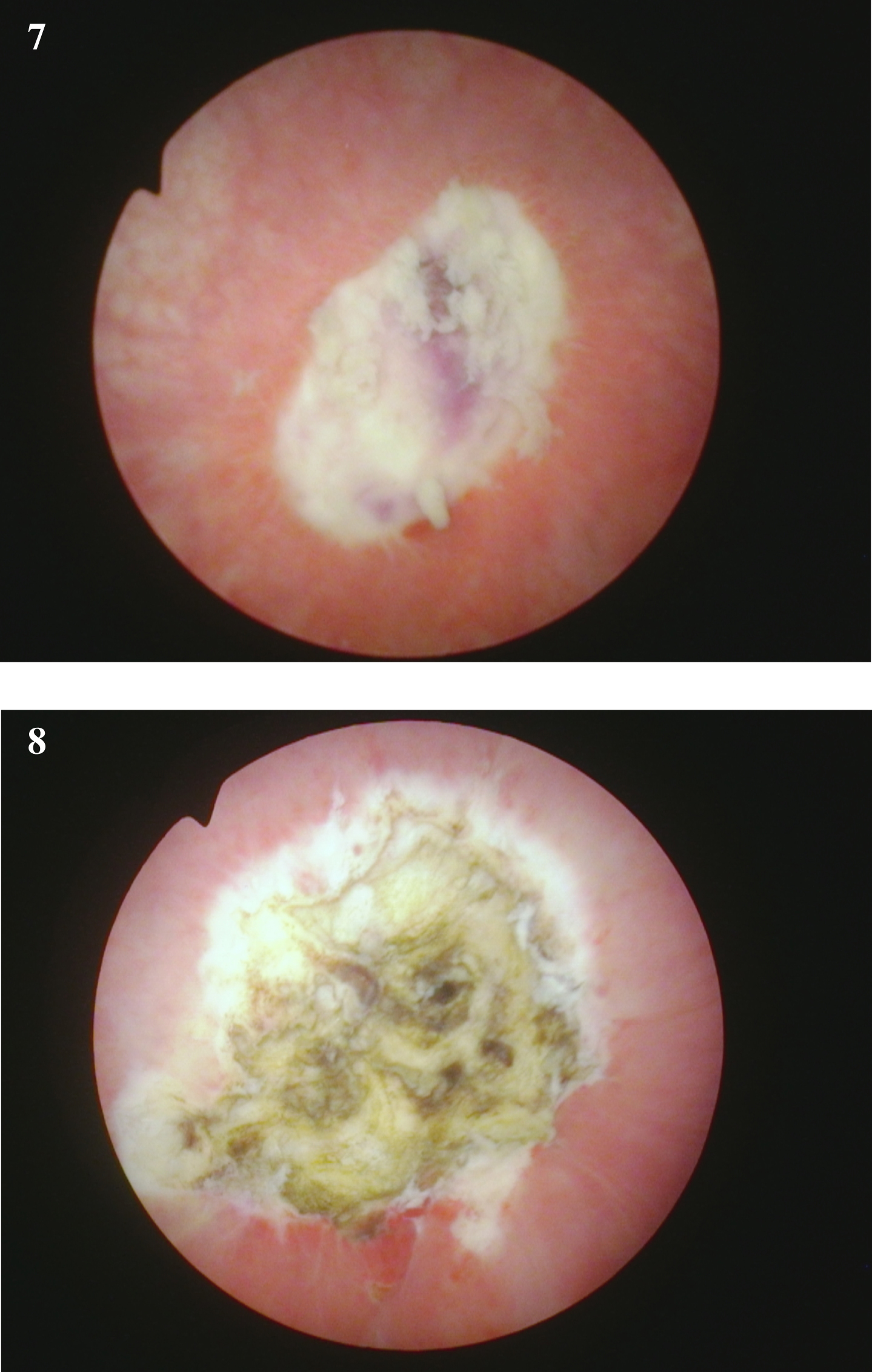
Fig. 7-8
Endoscopic view at the time of the repeat TUR BT following the initial diagnosis of a of the high grade T1 urothelial cancer. The rest of the bladder was normal.
She received intravesical BCG. A urine was sent for cytology at the time of the initial instillation. There were only a few atypical cells. She has no voiding complaints. The first follow up cystoscopy and cytology indicated no bladder cancer.
Of note there are no specific recommendations for “screening” of the genitourinary tract for patients with Lynch Syndrome. This woman saw me in 2021 because she wanted to have a cystoscopy given her awareness of an increased risk of a urothelial cancer.
I have had minimal experience with patients with bladder cancer who also had Lynch Syndrome and thus I asked two colleagues, Arthur Sagalowsky and Alex Zlotta, who have had an interest in urothelial cancer and Lynch Syndrome to comment on my case (their comments are following this report).
INFORMED CONSENT
Written informed consent for publication was obtained from the patient.
ETHICAL CONSIDERATIONS
The author has institutional review board approval for reviewing bladder cancer cases for outcome (MHS.2020.026).
CONFLICT OF INTEREST
Nothing to disclose.
Comments for Lynch Syndrome Case Discussion in the Journal Bladder Cancer
Arthur Sagalowsky M.D.
Professor Emeritus, Department of Urology, UT Southwestern Medical Center, Dallas, TX, USA
Mismatch repair (MMR) genes function to correct errors in nucleotide formation that occur during DNA replication. Abnormalities in specific MMR genes (MLH1, MSH2, MSH6, PMS1, and PMS2) create microsatellite instability (MSI), increased mutation rates, and a predisposition to several types of cancer. Lynch Syndrome (LS) patients have inherited errors in unique MMR genes which result in a particularly high rate of early age nonpolyposis colon cancer (HNPCC). Over the last forty years LS syndrome patients also have been noted to have increased rates of carcinoma of the endometrium, ovary, stomach, small bowel, pancreas, hepatobiliary tract, and of the upper and lower urinary tract. For practical and economic reasons, diagnosis usually is made only after genetic studies have been performed, just as in the current case, after a patient is found to have one or more cancers, and has a family history of multiple cancers in family members. Clinicians must note that apart from the large rate prevalence in HNPCC, the relative role that LS plays among patients with other forms of cancer is unclear. The literature mainly consists of case reports with small numbers of patients. Guidelines regarding treatment and follow up specifically of LS patients with cancer are not available.
The first, and perhaps most important thing for clinicians to note in the care of LS patients with any form of cancer, is that the abnormal DNA repair which defines the syndrome makes these individuals much more susceptible to the oncogenic risks of radiation than the general population. Imaging studies with radiation exposure (X-ray, CT) should be kept to a minimum in favor of ultrasound and MRI whenever possible. Radiation should be used in the treatment regimen only if there is strong evidence of superiority in efficacy compared to all other approaches.
The second point to consider is that of urothelial tumor location. The majority of early case reports of urothelial cancer in LS patients involved tumors of the upper urinary tract, i.e., the renal pelvis and/or ureter. Some authors stated there is no increased risk for urothelial bladder tumors. As the current case clearly shows, we now know that was an incorrect conclusion from insufficient case numbers. More frequent genetic testing in LS patients in the future should provide a better estimate of whether the proportion of urothelial tumors located in the upper tract versus the bladder is any different than for urothelial tumors in the general population.
Third, the presentation of this patient’s first urothelial bladder tumor has many features that portend a high risk for tumor recurrence and progression: solid, sessile architecture; large size; high grade; stage T1 proven lamina propria invasion. The promptly performed restaging TURBT one month later showed no residual tumor. No doubt a range of further treatment options was offered and discussed in detail: surveillance; intravesical therapy with BCG and or intravesical chemotherapy; primary cystectomy+/- neoadjuvant chemotherapy, chemo-radiation, to name a few. Anecdotally I must confess I have seen a few patients who had initially complete TURBT of similar large and high risk tumors who refused cystectomy due to advanced age or frailty and who remained NED following radiation therapy. I would not recommend radiation for this LS patient for reasons already stated above. It is not unreasonable that she began induction intravesical BCG therapy. However, she is at high risk for BCG failure. All can agree she needs prompt reevaluation for any gross hematuria. My personal recommendation for follow up during year 1 in this patient is
-
– urinalysis and cytology q 3 months
-
– office cystoscopy q 3 months
-
– Abdominal/pelvic MRI urography q3-6 months
-
– ask radiology if chest MRI is adequate vs CXR q3-6 months
If all studies remain negative for tumor recurrence, I would consider extending interval to q 6 months during years 2-3, annually in years 4-5. I also would consider alternating renal ultrasound versus MRI urography. I would urge consideration of radical cystectomy if there is any tumor recurrence. All of these recommendations are subjective and open to discussion.
REFERENCES
[1] Campbell-Walsh Urology, 9th Ed, 2007; 1:517-519.
[2] Watson P, Lynch HT: Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer 1993;71:677-685.
[3] Lynch HT, Ens JA, Lynch JF. The Lynch syndrome II and urologic malignancies. J Urol 1990;143:24-28.
[4] Leach FS, Hsieh JT, Molberg K, Saboorian MH, McConnell JD, Sagalowsky AI. Expression of the human mismatch repair gene hMSH2-a potential marker for urothelial malignancy. Cancer 2000;88:2333-41.
Risk of Genitourinary Malignancies in Lynch Syndrome Patients with DNA Mismatch Repair Gene Mutations in a Large Canadian Familial Registry
Karla Rebullara, Otto Hemminkia, Sean Skeldona, Kara Semotiukc, Melyssa Aronsonc, Cynthia Kukb, Katherine Lajkosza, Steven Gallingera, Zane Cohenc, Alexandre R. Zlottab
aSurgical Oncology, Urology, Princess Margaret Hospital, University Health Network, Toronto, ON, Canada
bUrology, Mount Sinai Hospital, Toronto, ON, Canada
cFamilial Gastrointestinal Cancer Registry, Zane Cohen Center for Digestive Diseases, Mount Sinai Hospital, Toronto, ON, Canada
Our group and others have previously described an increased risk for bladder urothelial carcinoma (UC) in LS patients with MSH2 mutations in addition to the well-known increased risk of upper tract disease.
We updated our initial findings and assessed the risk of bladder UC and UTUC in patients with MMR mutations analyzing a large cohort of LS patients within the Familial Gastrointestinal Cancer Registry (FGICR) in Toronto, Canada.
Patients with confirmed MMR mutations (MSH2, MLH1, MSH6, PMS2, EPCAM) were included and among mutation carriers, the majority had MSH2 (569) and MLH1(425) mutations. We used genitourinary (GU) cancer incidence rates on Statistics Canada, standardized incidence ratios (SIR) to compare cancer risk in LS patients diagnosed with GU cancer between 1992 and 2017 to the general Canadian population. We found that among the patients in the registry with a confirmed MMR mutation, bladder UC was found in 32 patients, 24 of which had MSH2 mutations (4.2%, SIR 14.4, p < 0.001) and UTUC was found in 25 patients, 18 of which had MSH2 mutations (3.2%, SIR 439.9, p < 0.001), both significantly above the expected rate in the general Canadian population. Noteworthy, in patients with both bladder UC and UTUC, over half (52.9%) of the bladder tumors occurred before the upper tract cancer, suggesting that bladder UC association to LS is independent of UTUC. Mean age of diagnosis was 59.1 and 62.2 for bladder UC and UTUC, respectively, with a male:female (M:F) ratio of 0.59 for both cancers, compared to a mean diagnosis age of > 70 and M:F ratio of 3 for both cancers in the general population.
At the time of the initial presentation, non-muscle invasive bladder cancer (NMIBC) accounted for 100% of all LS-associated UC (Ta was observed in 75% of cases), in sharp contrast with the 25– 30% of muscle invasive disease expected in most series of sporadic tumors. 43% of NMIBC were classified as low-risk, 21% were intermediate, and 36% were high-risk. 12/28 cases were high grade (HG) (5 TaHG and 7 T1HG). 39% of the patients had recurrences, and 6 of these patients had worse pathology (grade and/or stage) on recurrence. Median time to recurrence was 28.5 months (IQR38.5). With a median follow up time of 5.3 years, 18% of NMIBC patients progressed to muscle invasive bladder cancer (2 from the low-risk group, 1 from the intermediate group, and 2 from the high-risk group). Median time to progression was 36 months (IQR 42.3).
Our observations confirm a significantly increased incidence of bladder UC and UTUC patients with MSH2 mutation. Urothelial cancer was diagnosed at an earlier age in LS, and in contrast with sporadic cases, more common in females. Our finding also suggests a possible more aggressive clinical behavior of bladder UC in the LS population, as 18% of NMIBC patients progressed to muscle-invasive bladder cancer despite intravesical therapy. We strongly believe that urologists should be encouraged to recognize LS, and patients counseled about protocols to diagnose other Lynch Syndrome-associated malignancies. Genetic testing can benefit patients and family members, leading to earlier diagnosis, tailored surveillance, and risk reduction measures.

