This is an 83 year old man with a several month history of gross hematuria. He smoked cigarettes and cigars for approximately 50 years. His past medical history is pertinent for a coronary artery stent placed in 2009 and a stroke in 2019 from which he recovered without neurologic impairment. He has been taking the anticoagulant apixaban (Eliquis) since. He has congestive heart failure and hypertension for which he takes captopril (Capoten), carvedilol (Coreg), and furosemide (Lasix). His cardiac ejection fraction is 29 percent. His serum creatinine is 1.40 with an estimated GFR of 46 ml/min.
His physical examination was unremarkable with the exception of being moderately overweight, 205 pounds (BMI 31).
A CT scan of the abdomen/pelvis indicated a 5 cm infrarenal aortic aneurysm (Fig. 1). Importantly there was no hydronephrosis. The bladder contained a large solid mass (Fig. 2). There were no enlarged lymph nodes. A chest x ray indicated evidence of COPD and enlargement of the heart.
Fig. 1
CT scan identifying the abdominal aorta aneurysm (arrow).
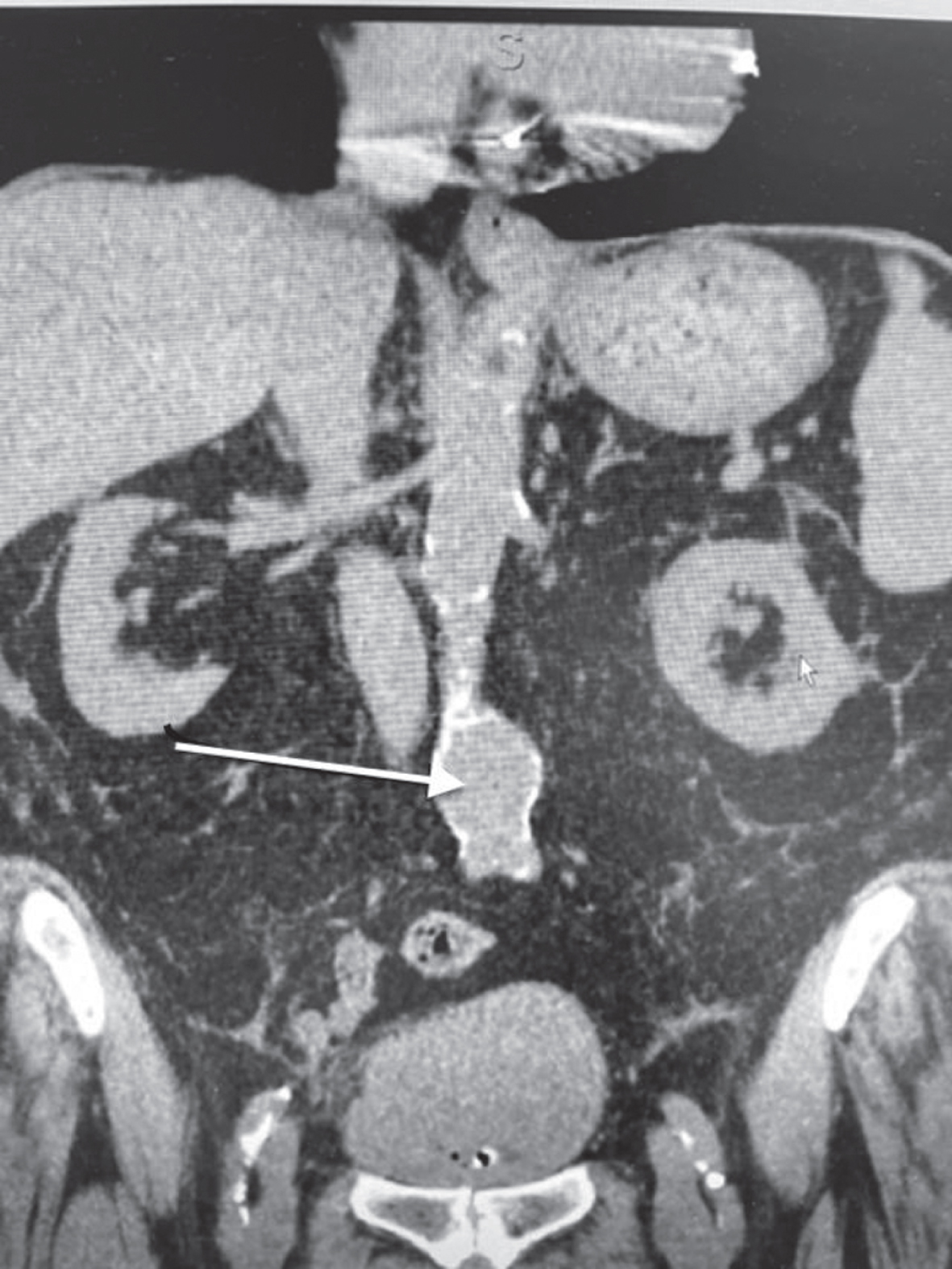
Fig. 2
The non contrast CT scan identifies a large bladder mass.
His urologist proceeded with a limited TURBT after identifying an extensive bladder tumor. The pathology indicated a mixed urothelial and squamous cancer of the bladder. Most of the specimen contained necrotic cancer. There was no muscle in the specimen.
I initially saw the patient in the outpatient office setting. Since he was not bleeding I proceeded with a flexible cystoscopy and confirmed the presence of an extensive, largely necrotic bladder mass which occupied much of the bladder.
With the intention to proceed with a more thorough endoscopy and possible TURBT under anesthesia I admitted him to the hospital where he was evaluated by a vascular surgeon and a cardiologist. The vascular surgeon recommended that the aneurysm should be treated with an endovascular graft. Following the performance of a series of cardiac studies, the cardiologist stated that the patient had no acute cardiac problems and could undergo cystoscopy and TURBT. My objective was to determine the size, location, and stage of the tumor in order to consider the management options.
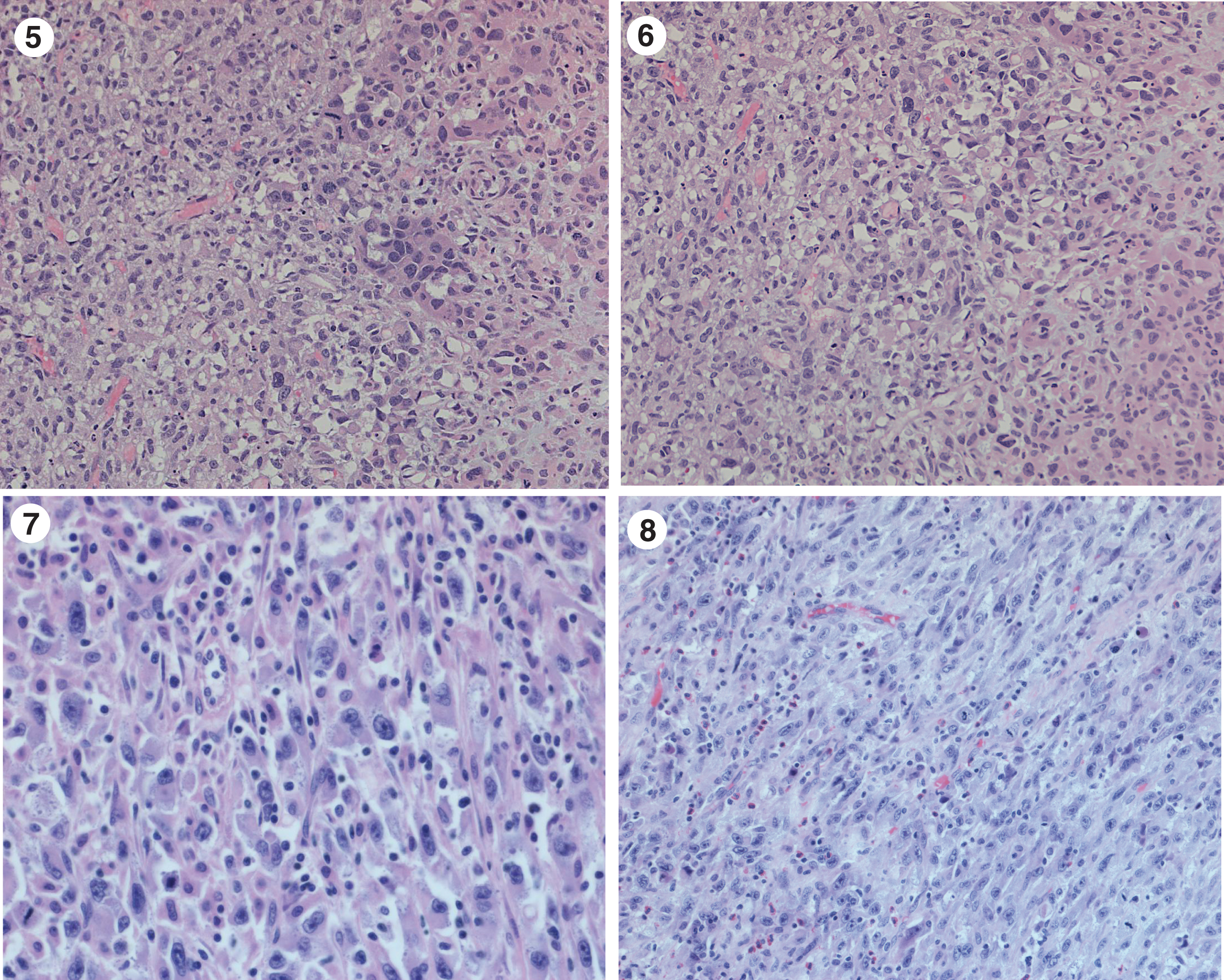
Once the patient was positioned following general anesthesia I performed a bimanual examination and did not appreciate a bladder mass however the accuracy was limited by his large abdominal girth. I proceeded with cystoscopy using a 70 degree lens to survey the bladder. Under anesthesia I gained a much better appreciation of the location and size of the bladder cancer. The ureteral orifices and trigone were normal. There was nothing to suggest carcinoma in situ (CIS). I identified a 2 cm sessile tumor to the left of the very large primary necrotic tumor mass which occupied the entire posterior wall (Fig. 3, 4). The smaller tumor was easily resected with the bipolar resectoscope. I began to resect the edge of the large mass and quickly realized that the risk of bleeding was far greater than any useful information I could gain by attempting any additional resection. The pathology indicated a high grade urothelial cancer with sarcomatoid differentiation (Figs. 5– 8). The tumor invaded at least into the lamina propria.
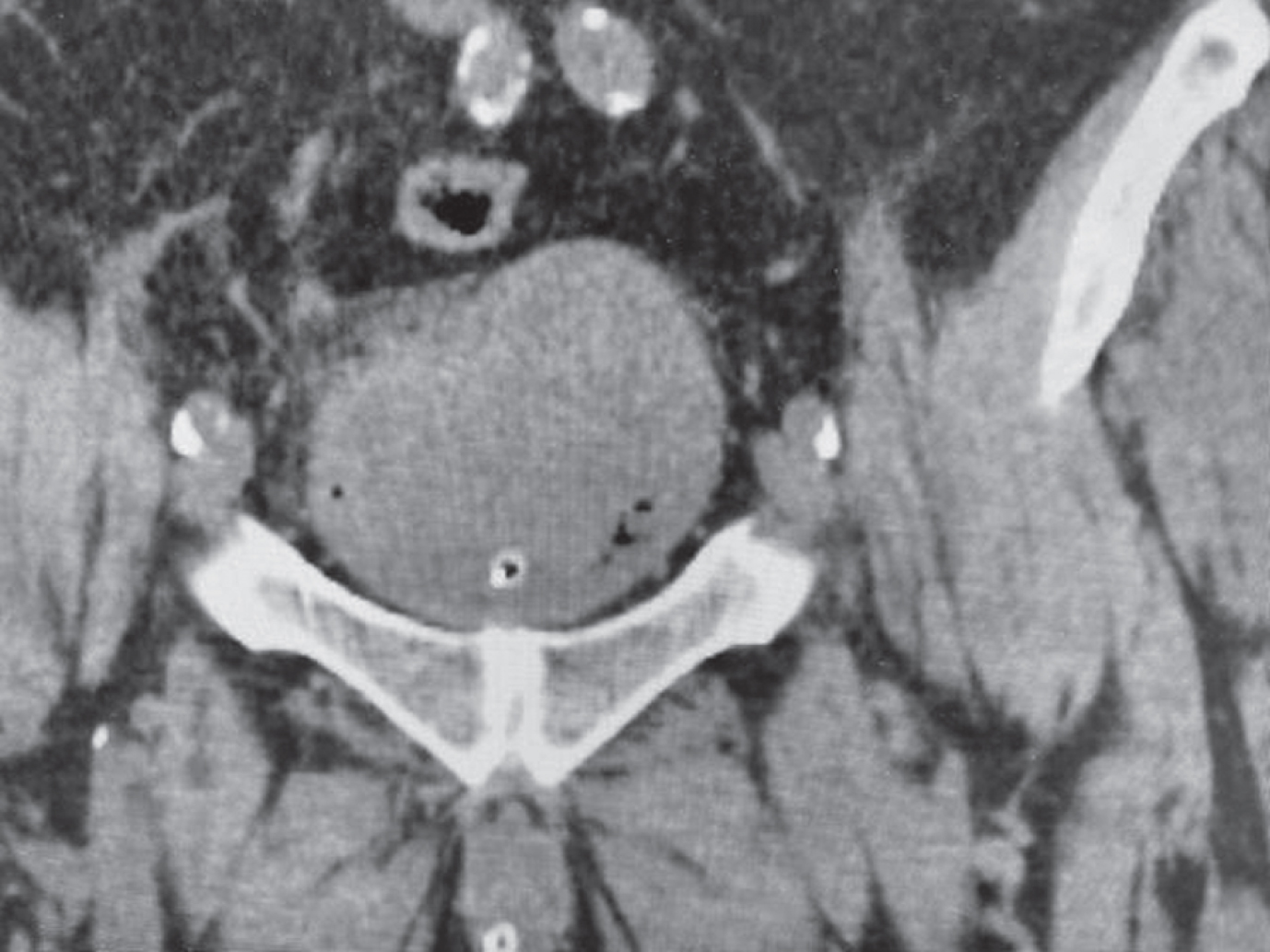
Fig. 3–4
Cystoscopic appearance of the extensive bladder cancer which appeared necrotic.
Fig. 5–8
Extensive urothelial carcinoma with sarcomatoid differentiation.
I discussed the patient with a medical oncologist to determine whether systemic chemotherapy was an option. He felt that given his age and diminished renal and cardiac function this would not be safe. Systemic immunotherapy might be a consideration if the bulk of the tumor was not a prohibiting factor and there was more mature data on its use as neoadjuvant therapy. The bulk of tumor was also a major consideration for not entertaining initial systemic therapy. There was some urgency in proceeding with treatment since the patient persisted in having gross hematuria.
The next decision was whether to perform a partial or total cystectomy. Ordinarily a cystoprostatectomy would be the standard approach. I thought that he probably could withstand the procedure although he would be at increased risk for perioperative morbidity. I had a lengthy discussion with the patient and his family. I decided to consider a partial cystectomy if the intraoperative findings warranted this approach. Based on the endoscopic appearance, I thought that the trigone and enough of the bladder was normal so that I might be able to remove the tumor and leave sufficient bladder capacity to allow “normal” voiding. Given the adverse pathology, I felt his prognosis would not be dependent on the extent of surgery.
The vascular surgeon opined that the endograft should be placed prior to any abdominal surgery and this was successfully performed.
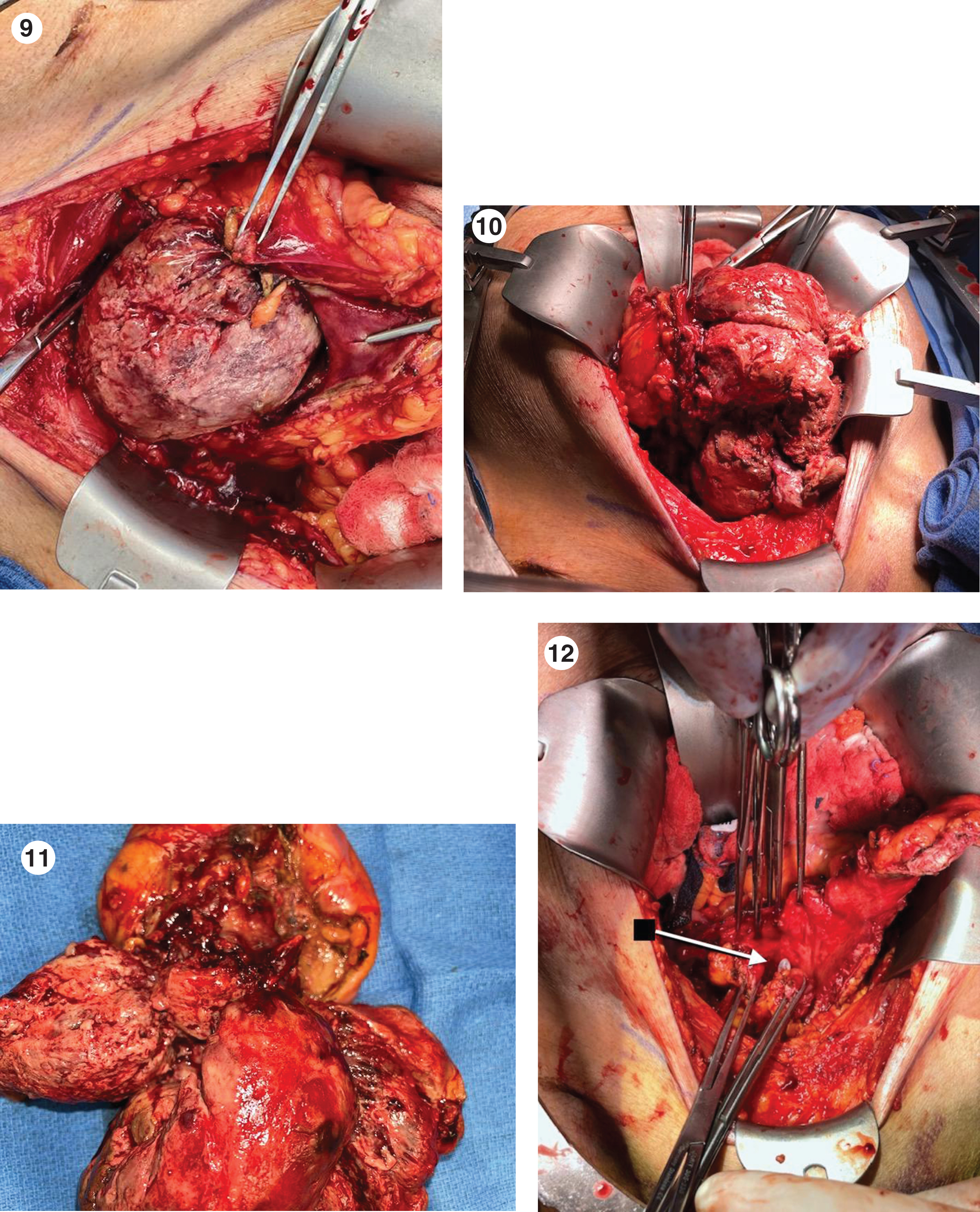
Two weeks later I mobilized much of the bladder and opened it anteriorly. I was astounded by the bulk of this cancer (Fig. 9– 12). I performed a partial cystectomy. The pathology was a muscle invasive high grade undifferentiated urothelial carcinoma with sarcomatoid and squamous differentiation. The cancer extended to the soft tissue at the posterior bladder surface (pT3a). Four lymph nodes were negative. Due to the recent graft placement only a minimal lymph node sampling was performed. There were no enlarged nodes.
Fig. 9–12
Opened bladder with a very extensive mass which invades and occupies the entire posterior portion of the bladder.
The patient is recovering uneventfully and has no voiding problems and no hematuria.
He will be monitored with standard imaging.
The criteria for a partial cystectomy rather than a total cystectomy for bladder cancer traditionally includes: 1. A solitary tumor in a location which allows a two cm negative margin, 2. No previous bladder cancer, 3. A muscle invasive bladder cancer since Ta/T1 cancers can be treated with a TURBT, 4. Negative bladder biopsies to ensure no CIS in the normal appearing bladder, and 5. Sufficient remaining bladder to allow for an adequate bladder capacity. This man met some, but not all, of these criteria. Sometimes we use our judgement given a particular clinical scenario and not adhere to well-conceived guidelines. This is such a case. Time will tell if I made the “correct” decision.
Given the increasing response rate with neoadjuvant systemic chemotherapy and immunomodulators, some urologists have proposed expanding the use of partial cystectomy for cases in which there is a solitary cT2-3 urothelial cancer in a location which would allow adequate margins and in which there appears to have been an excellent response to one of these systemic therapies. Not infrequently there are patients who have what appears to be a complete response, i.e. negative cystoscopy, cytology, and possibly biopsy, after neoadjuvant therapy. We know that 30% of patients who receive induction systemic therapy do not have residual bladder cancer following a radical cystectomy. When the initial tumor is in a location that would permit a partial cystectomy is this not a reasonable approach?
AUTHOR CONTRIBUTIONS
MSS contributed to the work conception, performance, analysis of data and writing; NAA contributed to performance and analysis of data.
INFORMED CONSENT
Written informed consent for publication was obtained from the patient.
ETHICAL CONSIDERATIONS
The author has institutional review board approval for reviewing bladder cancer cases for outcome (MHS.2020.026).
CONFLICT OF INTEREST
MSS and NAA have nothing to disclose.

