21 May 2020
Amsterdam, NL – Bladder cancer is associated with significant illness and mortality, particularly if treatment is delayed. Writing in the journal Bladder Cancer, researchers have outlined recommendations for treatment of both muscle invasive (MIBC) and non-muscle invasive (NMIBC) bladder cancer during the COVID-19 pandemic based on data from trials and prior studies, and taking into account the current strains on the healthcare system.
The COVID-19 pandemic has disrupted the paradigm of healthcare in many ways. Hospital resources are stretched thin as the number of cases continue to climb daily. In cancer care, treatment must be weighed against the issues of viral transmission, resource utlization, healthcare access, and community safety.
 "The COVID-19 epidemic has forced doctors to prioritize patients with time-sensitive illnesses and defer those with conditions that can wait. We know some patients with bladder cancer simply can’t wait on treatment without compromising their oncologic outcomes," explains senior author Lambros Stamatakis, MD, Director, Urologic Oncology - MedStar Washington Hospital Center, Assistant Professor of Urology - Georgetown University, Washington, DC, USA. "Our hope with this paper is to provide a framework that can help clinicians navigate treatment decisions for their bladder cancer patients in the setting of the COVID-19 epidemic."
"The COVID-19 epidemic has forced doctors to prioritize patients with time-sensitive illnesses and defer those with conditions that can wait. We know some patients with bladder cancer simply can’t wait on treatment without compromising their oncologic outcomes," explains senior author Lambros Stamatakis, MD, Director, Urologic Oncology - MedStar Washington Hospital Center, Assistant Professor of Urology - Georgetown University, Washington, DC, USA. "Our hope with this paper is to provide a framework that can help clinicians navigate treatment decisions for their bladder cancer patients in the setting of the COVID-19 epidemic."
First author Filipe L.F. Carvalho, MD, PhD, MedStar Georgetown University Hospital, Washington, DC, USA, adds: "Many institutions have stopped performing elective surgery and outpatient procedures during the COVID-19 epidemic. This can be problematic for patients with NMIBC who need frequent cystoscopies, bladder biopsies/tumor resections, and intravesical therapy. For patients with MIBC, delay of treatment may unfortunately result in missing the window of opportunity for cancer cure."
Risk stratification is key in helping clinicians manage this cancer. The authors note that several studies recommend that patients with low risk NMIBC can be safely managed with active surveillance after transurethral resection of bladder tumor (TURBT), generally the first treatment for bladder cancer. "Given the inevitable shift of resources diverted to treat patients with COVID-19, and to prevent viral exposures during medical visits, we propose surveillance for all patients with a history of low risk tumors, and those with non-high-grade intermediate risk NMIBC, reserving TURBTs for symptomatic patients," says Dr. Stamatakis.
On the contrary, patients with high risk NMIBC should proceed with active treatment. Since hospitalization after TURBT is uncommon, and the risk of aerosolization of virus during these procedures is low, the authors recommend that these surgeries should be continue if possible, within a hospital system.
MIBC is a more lethal disease, and studies show that delayed treatment leads to significantly worse outcomes. One of the standard therapies is radical cystectomy with urinary diversion. However, the authors observe that the COVID-19 pandemic has forced the urologic community to reconsider standard practices. Radical cystectomy is resource intensive, with the need for ventilators, masks, and other equipment. Moreover, the need for human resources is extensive when a hospital system is performing major surgeries. These factors all need to be considered during this period when material and human resources are scarce and may be needed elsewhere.
The authors believe that radical cystectomy is one of the procedures that should be prioritized during the COVID-19 epidemic. They recommend that the choice of treatment for MIBC should be individualized, with specific consideration given to patient symptoms, tumor volume, access to cancer treatments such as infusion centers and radiation centers, access to post-hospitalization care (i.e., rehab/skilled nursing facilities), and the current status of the virus in the community. They note that enhanced recovery after surgery protocols should be implemented to allow for improved convalescence. Telemedicine should be feasible for pre- and postoperative visits in most instances.
Currently there are multiple clinical trials available for patients with NMIBC and MIBC, however, recommendations from governments and other institutions regarding these trials are evolving. The authors recommend that for patients already enrolled in therapeutic trials, all efforts should be made to continue providing the appropriate treatment if safe for the patient and local healthcare community.
While non-therapeutic trials focused on biomarker discovery or relying on tissue banking should be placed on hold, “we encourage clinical investigators to consider novel approaches to patient monitoring and disease management. Looking forward, recommendations will inevitably need to evolve with the quickly changing landscape of medicine during the COVID-19 epidemic,” comments Dr. Carvalho.
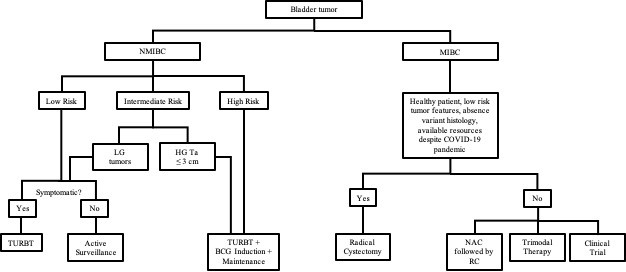
Caption: Treatment algorithm for patients with bladder cancer during the COVID-19 pandemic
###
NOTES FOR EDITORS
Full open access article: "Considerations about Non-Metastatic Bladder Cancer Management During the COVID-19 Pandemic" by Filipe L.F. Carvalho, Lan Anh S. Galloway, Ragheed Saoud, Piyush K. Agarwal, and Lambros Stamatakis (DOI: 10.3233/BLC-200311) published online in Bladder Cancer ahead of publication of Volume 6, Issue 2 (scheduled for June 2020) by IOS Press. The article is openly available at: content.iospress.com/articles/bladder-cancer/blc200311.
Contact
For additional information contact Diana Murray, IOS Press (+1 718-640-5678, d.murray@iospress.com). Journalists who wish to interview the authors should contact Lambros Stamatakis (lambros.stamatakis@medstar.net).
About Bladder Cancer
Bladder Cancer is an international multidisciplinary journal to facilitate progress in understanding the epidemiology/etiology, genetics, molecular correlates, pathogenesis, pharmacology, ethics, patient advocacy and survivorship, diagnosis and treatment of tumors of the bladder and upper urinary tract. The journal is dedicated to providing an open forum for original research in basic science, translational research, and clinical medicine that will expedite our fundamental understanding and improve treatment of tumors of the bladder and upper urinary tract. bladdercancerjournal.com
About IOS Press
IOS Press is headquartered in Amsterdam with satellite offices in the USA, Germany, India and China and serves the information needs of scientific and medical communities worldwide. IOS Press now publishes more than 80 international peer-reviewed journals and about 75 book titles each year on subjects ranging from computer science, artificial intelligence, and engineering to medicine, neuroscience, and cancer research. iospress.com


